Chapter 12, The Informed consent process
Table of content
The Informed consent is the cornerstone in protecting study participants and their rights and safety. Developed still after the WW2, the Nuremberg code defined for the first time the main principles of the ethical conduct of research on humans.
Here is the structure of the Informed Consent Process chapter from the CRP3.0 course .
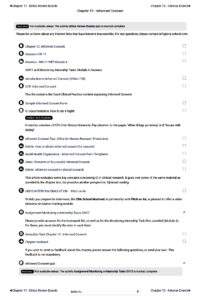
All chapters have been conceived in the same way – with PDF files, videos, workshops and other presentations, samples of Canadian and US documents, checklists to use in the future work without missing anything, even the smallest detail, job search coaching and networking hints, professional branding support, etc.
See an excerpt of the chapter 12, detailing the GCP requirements for the process of obtaining Informed Consent.
Next >>
As seen in the Table of content, our course provides several additional files and external sources to better prepare the students for interviews. Additionally to that, specific CV adaptation and interview preparation are provided for every vacancy for unlimited duration (till hired) without additional costs.
The objective is to provide the maximum of information to students, so that they see the same topic, presented by different sources, see the common points and get fully the context of all activities, master the industry slang, and transform it into active vocabulary to use during networking and interviews.
The remote internships provide experience in the tasks, needed for the activities required for all the roles, and mainly those of the Study Coordinator, working in the clinical sites as Assistants of the PI (Principal Investigator), and the CRAs, acting as monitors (inspectors) for the Sponsor or a CRO (Contract Research Organization), as well as for all the other roles, involved in the Study Design, Organization and Start-up, Data Management QC, QA of the Clinical Site compliance, etc.
Our goal is that students start speaking like experienced seasoned professionals, without searching for the right terms or acronyms, position themselves in the best possible way, network successfully and succeed the interview to get a first job.
After that, career growth is automatic. Once you are in, you are in forever. In about 4-6 months recruiters will discover that you have enough practical experience to move to higher-level jobs.
They will start offering you to jump ship, so that they get their commissions. Or the employer will promote you because somebody has been solicited by a recruiter and left the organization. It is easy, it takes only 2 weeks notice. With the high shortage of experienced staff, people change jobs every 1-2 years to go up.
The Nuremberg Code principles became the basis of all subsequent clinical trials regulations and laws, where the Informed Consent is the first, and utmost important requirement for conducting research on humans with approved and non-approved medicinal products and treatments with known or unknown adverse effects.

