Since 2009 we provide worldwide online clinical research certification & job placement support
Click here to see the latest news

For safety reasons, face-to-face consultations are not offered anymore in the Academy since May 2020 (legal address only, no admission).
For a FREE 1-hour 1-on-1 Career Consultation on your eligibility, CV adaptation, and Action Plan, suitable for you, contact us by email at info@cra-school.com, by Skype at CRA School Montreal, or by phone from 9:00 AM to 9:00 PM EST (UCT-5), and by Skype chat – till midnight.
By phone: multilingual, 7/7 LIVE Support : +1 (514) 257-3003
WhatsApp: Support 7/7, till 9:00 PM EST : +1 (514) 961-9351
Skype: by chat, 7/7, till 11:30 PM EST @ CRA School Montreal
To call at the right time, use this Time Zone converter
“An investment in knowledge always pays the best interest”
. Benjamin Franklin
Education is the best investment, and to get a diploma in the comfort of your home became the new standard of learning. Already 12+ years the CRA School offers worldwide an Accredited Clinical Research Online Certification PG program with 3 remote internships in the Academy & 7/7 LIVE SUPPORT + Placement Assistance Till Hired to help students enter this ever-growing field. 1-on-1 courses for improving oral English or French are provided by Skype on request 7/7 at additional, especially reduced fees for students. Vaccine trials created a huge demand for more staff. In the 2-nd year, most of our alumni are at $80,000/yr, and several are at rather high-level roles today. See them on LinkedIn.
* * *
You think that you know GCP? Check do you know the GCP basics?
*NB: This is not an admission test, the course is open to everybody
The Accredited Clinical Research Post-graduate Certification program CRP 3.0 covers the Canadian, US, and international GCP regulations for clinical trials conduct. It is designed to support till hired professionals in career change, newcomers and aspiring Canadian Permanent Residents, who plan to settle in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, and the Atlantic provinces, by preparing them in advance to get a job. The special fee reduction is our contribution to alleviating the pandemic burden.
The combined CRP 3.0 PG course prepares students not only for the CRA role, but also for almost all jobs in this field that don’t require a license to practice, including CRC, SSU, CTA, RA, DM, and even CPM, SSM, DSA, PHV, MSL, etc. It is internationally accredited by CPD UK and recognized by TransCelerate BioPharma USA as satisfying all the requirements for mutual recognition among clinical trials sponsors of GCP training courses.
Individual, self-paced, ONLINE program with unlimited 7/7 LIVE support, adapted to the goals of every student. A lot of home-based jobs, excellent for young mothers. Share the news with friends, who face career change, but lack the necessary tools & support, and get a $100 Referral bonus if anyone, whom you informed about us, enrolls someday
N.B.: If you plan moving to Canada, just join us 6-7 months before your flight and arrive JOB READY with a Canadian practical experience from the 3 internships, a Canadian diploma + a network of contacts + unlimited support till hired. This detailed ONLINE program allows to resolve the obstacle of the lacking Canadian experience, to arrive eligible for interviews, and get quickly a job. Share the news <more>
.

* PATH to Clinical Trials Jobs for international graduates:
Clinical Trials are a great alternative for foreign-trained RN, MDs, BDS, DVM, PharmB/D, PhT & BSc/MSc/PhD degree holders. (No need for recognized diplomas, qualification exams, nor license to practice):
* Regulatory Affairs, Data QC/QA, Drug Safety & Pharmacovigilance Jobs
* Medical Affairs roles, high wages, fast access (4-6 months detailed self-paced training with 3 remote monitoring internships & 7/7 LIVE Support till hired)
*Most jobs became home-based today. Interesting possibilities for young mothers with babies. Frequent promotions due to the chronic 30% shortage of experienced staff. See more here
* Main obstacle to get into the industry:
The law requires regulatory training AND prior experience (at least from an internship). We provide both + unlimited support till hired. Self-paced study with expected max. duration – up to 2 years. After that exams must be redone.
* What is different in CRP_3.0:
To make students eligible for interviews, in parallel to the regulatory ICH GCP E6(R2) course, 3 remote part-time non-paid monitoring internships on old studies are provided in the Academy, plus:
+ active networking support to get job leads for the “hidden jobs” that are never posted, but filled-in by references,
+ unlimited assistance in the CV adaptation for every role + interview practice and preparation in advance (till hired).
* Who is eligible:
– Candidates worldwide with nursing or any university degrees
– Research assistants, interested to join this rewarding industry
– IMG in career change (MD, DDS, DVM, PharmD, BPharm, RN)
– Clinical Nurses & CRC, looking to jump ships to the industry
* What you will get:
– International PG Diploma of Certified Clinical Research Professional (CRA, IH-CRA, CTA, CRC, DM, PM). All RA roles are covered
– “Hands-on” experience in Canadian/US regulatory environment from 3 monitoring internships, incl. Study Management Software
– Unlimited 7/7 support till midnight EST (UCT-5) from our tutors (NB: we are not a placement company, we assist you till hired)
* Who will profit to enroll:
IMG (RN/MD/DDS/DVM/PharmD/BPharm/MSc/PhD) and any life-sciences graduates worldwide. (No study permit needed. Private studies, not funded by government. Remote 100% ONLINE course, does not provide student’s visa support).
*
Demo Chapter 12, Informed consent
Click here: >>>
Global Accessibility Program (GAP)
.
In the frame of the Global Accessibility Program (GAP)
an additional regional discount is offered for some
low-income countries in Africa, Asia and Latin America
To enroll, interested candidates must send their CV with
their current home address and their official names as per
their ID to <info@cra-school.com> to verify their eligibility
for the GAP program and to send them a payment invoice.
The concept of our program is totally different. Self-paced
individual (not in a group), starts on the day of enrolling.
Provided worldwide, 100% ONLINE course, with 7/7 direct
contact with a tutor from 9.00 AM till midnight EST (UCT-5).
This flexibility allows to study while working full-time.
The 3 remote part-time monitoring internships are done in
3 Phase II, III & IV studies on metabolic syndrome, LDL-C
and diabetes in the Academy, in parallel to the GCP course
and have a duration of 6 months or more. Support till hired.
.
* FREE 1-ON-1 CONSULTATIONS *
.
Do you want to work in clinical research in Canada or
to move to Canada? Are there jobs available? How are
they paid? Are you eligible for them? Where to start?
Do I fit? You probably have hundreds of questions.
For a FREE 1-hour LIVE consultation, just say “Hi” by
Skype to our account “CRA SCHOOL MONTREAL” or
book a time spot 7 days/week here. Reserve 1 hour time.
Get answers to all your questions directly by our expert
Discuss in detail the amazing careers opportunities
in the rewarding and booming clinical trials industry
and what Action Plan would be suitable for you.


Well-known fact: Nurses make great Clinical Research Coordinators (CRC) and Clinical Research Associates / Monitors (CRA). They are preferred for CRC & CRA roles, and plenty of other roles too, because they know the principles of ethical clinical practice, the medical terminology, physiology and pathology, diseases and treatments, and how clinics operate.
In Regulatory Affairs (RA), no medical acts are performed, therefore no license to practice in the country is required. Work with data and documents, organization and control of clinical trials. Well paid jobs, no body fluids, no night shifts, no exams. A FAST TRACK road to the profession for foreign trained nurses.
The Accredited Postgraduate Program CRP3.0 provides not only GCP certification, but also practical experience in the clinical trials related tasks, required by law, active support in building a network of contacts with hiring managers, assistance in CV adaptation for every role, plus unlimited interview preparation till hired. The concept is unique and not offered by other training providers.
Contact us for details on how to kick-start your clinical research career. Get 7/7 LIVE Support by an individual tutor till getting the first job in the rewarding clinical trials industry. Proof of qualification required at registration. Foreign diplomas accepted.
SPECIAL for foreign and Canadian nurses: CAN $100 off regular (non-GAP) fee with the code ICRANURSES
To apply, email your CV to info@cra-school.com to assess your eligibility, and to suggest you a suitable Action Plan.
Become an Affiliate: just share the info with friend
With the chronic 30% shortage of experienced staff, some 80-90% of the jobs are
never posted (especially the entry level jobs), but filled-in by internal promotions
and references. Companies pay a referral bonus of $1,000 up to $2,000 if they hire
the candidate that you referred. Their employees are interested to bring your CV
to the hiring manager, but they don’t know you. Hence the need to network.
As jobs are “hidden”, candidates don’t see them and these rewarding well-paid
Regulatory Affairs jobs in the booming clinical trials industry are widely unknown.
Don’t hesitate to share this info with friends, who may profit from this opportunity.
.
Refer a friend and get a Referral Bonus of $75 ($50 for GAP)
.
Inform friends & colleagues about these highly-paid careers in chronic shortage
of staff, our free monthly Career Events, and our Clinical Research PG Diploma
On-boarding program with 3 internships and Placement Support till Hired (PATH),
and get a $75 referral bonus for anyone who eventually enrolls in the program.
To register a Referral, email us the names and email addresses of the person
whom you informed.
Bonuses are paid at the beginning of the month after the candidate enrolled.
The number referrals is unlimited, don’t hesitate to spread the news.

.
Electronic Data Capture (EDC) & Clinical Trial Management
Software (CTMS) training and internship are an integral part
of the CRP3.0 Post-Graduate program. This is a major hiring
requirement. It is capital to have such practical experience.
Vaccine trials hasten the need for new clinical sites & staff.
They need 40,000+ participants, 10 times more than drugs.
This means a lot of new staff to handle this huge workload.
There have never been that many new jobs in this field. It is
the best time to go to this rewarding & blooming industry.
The CRC, CTA, & In-house CRA roles are great entry-doors to
the clinical trials field as they don’t need much prior experience
The demand of experienced staff is always high & will only grow.
Share the information with friends, who will fit and can profit.



























Services
|
CRA School |
Others |
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
What is new: |
|
|
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
And also: |
|
|
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
|
✔ |
✘ |
Unique approach
Get a job, not just a diploma
1) Fast access to well-paid and in-demand careers
2) International: All clinical trial careers covered
3) Internships for hands-on Canadian experience
Interview preparation
Job-specific preparation for interviews
1) Job description analysis + CV adaptation for every job
2) Preparation for every interview until hire
3) Training in advance for topics not yet covered
Professional branding
Coaching & support on Professional positioning
1) Professional branding coaching & mentoring
2) Reference letters, job alerts & job assistance
3) Networking meetings & weekly consultations
Full accessibility
Accessible when you need it
1) Online training to advance at your own speed
2) 7/7 LIVE Support by Skype till late evening
3) Flexible payment options adapted to your needs (2, 3 or 6 installments)
Job insertion support
Active Job Placement Assistance
1) Instructions on pre-selection questionnaires
2) Coaching on typical interview questions & traps
3) Introduction to industry slang & terminology
...AND more!
International program for all needs
1) US, Canada, EU, ICH GCP & QC regulations
2) Options for GCP professionals, MD, MSc/PhD
3) Referral Program with unlimited bonuses
About Us
The International Clinical Research Academy (the CRA School) is the only provider of international clinical research training with internships and job-placement support (Placement Assistance Til Hired; PATH program).
Our program is suitable for candidates with life science background including RN, BSc, MSc, PhD, MD, PharmD, GCP professionals, corporate staff, IRB/REB members.
Our training is universally-applicable and provides students with a solid theoretical and practical base to work in any country.
Our Mission
Our objective is to provide the knowledge and necessary tools that will facilitate access to rewarding careers in clinical research, including Clinical Research Associate (CRA), Clinical Research Coordinator (CRC), Clinical Trial Assistant (CTA), Study Start-Up Assistant (SSU) or Regulatory Affairs (RA).
Our courses provide the building blocks that lead to other fields including Data Management (DM), Clinical Project Management (CPM), Drug Safety Associates (DSA), Pharmacovigilance (PV), Medical-Science Liaison (MSL), etc.
Roles in clinical trials
Regulatory agencies (FDA, Health Canada or other) inspect only a small part of all studies (around 2%). The Sponsor company or individual investigator who initiates a study are obliged by law to periodically monitor the quality of the generated data and ensure that the rights and safety of the study participants are protected. The physicians (PI or sub-investigators) are responsible for the evaluation of their patients and the quality of the data
Data management, quality control & statistical treatment of study data are done by sponsor’s staff, Clinical Research Associates (CRA), in-house Monitors, Data Managers (DM), Drug Safety Associates, Medical Advisers, Biostatisticians, Project Managers and other staff trained on the study protocol, clinical trial regulations and Sponsor’s SOPs.
What is a CRA/CRC?
The Clinical Research Associate (CRA) is a monitor (inspector) who periodically visits the study sites to supervise and inspect the work of the clinical investigators and their coordinators. The CRA verifies their compliance to the protocol and the regulations, makes sure that all regulatory requirements are met and the quality of the data is acceptable. The in-house CRA or the Clinical Trial Assistant (CTA) organize all study-related activities.
The Clinical Research Coordinators (CRC) or the Clinical Research Assistants work in the medical clinics (called sites) where the studies take place. They organize all interactions with the study participants and all the study-related activities at the site. The Principal Investigator (PI) is responsible for the patients’ care, evaluations, and the conformity of all the study conduct with the law and clinical trial regulations in force.
Eligible Candidates
Companies look for good communication skills in English and university diploma, which guarantees that the candidate will be able to understand a research protocol when trained. Life sciences, health care, nursing, biology, biochemistry, biomedical engineering or allied health field are preferred.
For some high-level jobs, an advanced scientific degree is required. Prior experience is always the 1st hiring criterion. The best hiring chances have professionals with some prior practical experience in clinical research activities in N.American regulatry environment.
International candidates
Health professionals trained abroad often have difficulties when they move to another country. Foreign diplomas may not be recognized and the required local practical experience is missing.
In clinical trials, except for the clinical investigators and their nurses, all the other Clinical Research Professionals do not perform medical acts. They only work with the study data, the clinical trial organization, data quality control and documentation. Therefore, they don’t need any license to practice in the country. Today most jobs became home-based and are done remotely
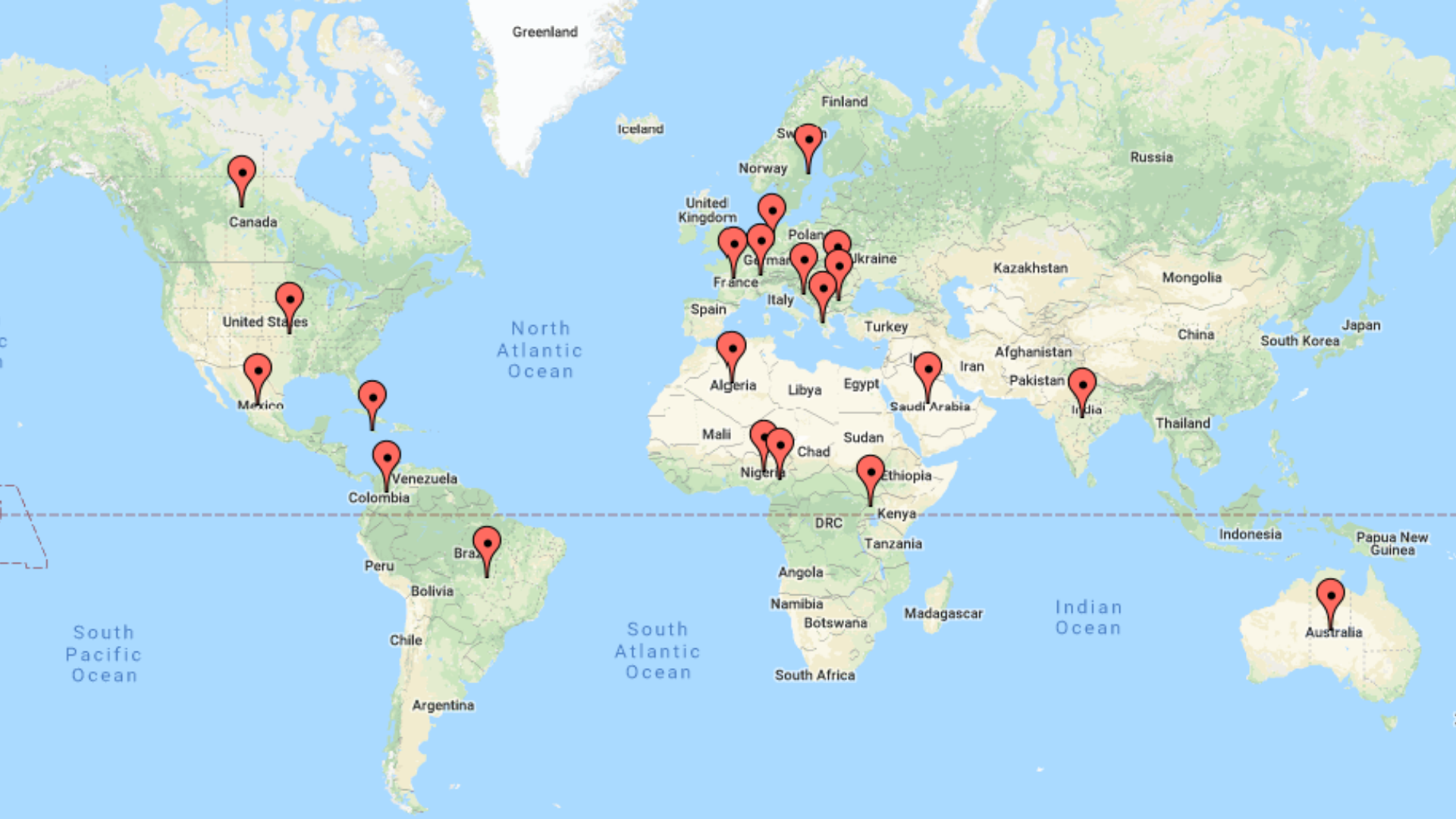
Consultants and Representatives
Clinical Research Advisor
Ken Boudreault,
Senior CRA II
Synteract, Montreal
Consultant & Mentor
20+ years experience
Data Management Advisor
Nassima Benmouffok
Ass. Director of Data Management,
Ethica CRO, Montreal
Senior Data Manager
Medical Science Liaison Advisor
Thomas Rolain,
PhD, CCRP/CCRA
Syneos Health
SrCRA and MSL
Pharmacovigilance and Drug Safety Advisor
Medical Writing Advisor
Sefika Ozturk, PhD
Medical advisor
MSL at Merck
Clinical Trials Management Advisor
Radia Ouelaa, PhD
Clinical Team Manager, PPD
Onco/Hematology
Clinical Research Management
Janet Vergara, SrCRA
Ass.Director at Merck
Clinical Research Manager
Clinical Trials Advisor, Trainer and dedicated Mentor
Bahareh Najjar SrCRA
MedPace, Montreal
CRA Mentor and Trainer
Clinical Operations Advisor
Anna Leonov, PhD
Clinical Operations
Syneos Health
Regulatory Affairs Advisor and Mentor
Mona Ramezani
IH SrCRA at Merck
Regulatory Affairs
Medical Devices Advisor
Monir El Azzouzi,
CEO and Consultant
Easy Medical Device
Medical Writing Advisor and dedicated Mentor
Dr Anand Devasthanam, PhD
Ass. Medical Director
Syneos Health, Boston
France
Karima Kezadri, MBA, MSc, CCRP
Regional Rep. for
Maghreb & MENA
Tel: +213559918058
hopekarima@yahoo.fr
Nigeria
David Dele-Davids
Lagos, Nigeria.
Tel:+2349093309249
WhatsAp: +19495202173
deledavidsconsulting@gmail.com
India
Monisha Sundar
Ontario; Canada
New Delhi; India
Tel: +16472387293
+918427688701
mishu_shiv@hotmail.com
Bulgaria
Dr. Jamil Rashed
Sofia, Bulgaria.
Tel: +359879450847
Djamilrashed@gmail.com
AUSTRALIA
Jyothi Surada,
BPharm, CCRP
CRC,
BC
Benard Kimwei
Burnaby, BC
Tel: +1 403 465 6900
benard.kimwei@gmail.com
AB
Olaniyi Taiwo
Edmonton, AB
Tel: +1 306 450 2974
sotaiwo06@gmail.com
MB
Dina Tabatabaei, MA,
CCRP, CRA School Rep
Brandon, MB
(416)879-7575
dtabataba88@gmail.com
USA
David Dele-Davids
Baltimore, MD, USA.
Tel: +1(949)520-2173
deledavidsconsulting@gmail.com
UGANDA
Daniel Okodan, MSc,
CCRA, CRA at PPD
Entebbe, Uganda
okodandaniel@gmail.com
DUBAI
Taline Sagherian, RD
CCRP, Dubai, UAE
+(971) 50 935 4208
sagheriantaline@gmail.com
FIJI
Oluwafemi Ojo, MD, CCRA
Physician, Tawua, Fiji
drfemiojo@gmail.com
GERMANY
UGANDA
Sylvia Namanda,
BSN, CCRP,
Makarere University
(+256) 756 762 005
sylvian531@gmail.com
IVORY COAST
ISSATOU DIALLO, CRA
Regional Manager
Afrika at MCT-CRO
issatoubella@gmail.com
Medical Writer
Christina Sanguinetti,
Medical Writer, CEO
LaconicMed, ON
christina@laconicmed.ca
Recent News:
One rewarding day in the life of a CRA – tasks and challenges, free conference of Ken Boudrault, SrCRA
One rewarding day in the life of a CRA – tasks and challenges, Free Web Conference of Ken Boudrault, SrCRA and mentor, organized by CRA School Montreal
Covid-19 hastens the need for new clinical trials, staff & sites, both in the industry and in academic settings
Covid-19 hastens the need for new clinical trials, staff & sites. Join our Global Networking Series to explore the Online Approval Process of clinical trials
Clinical Trial Workshop with Ken: Clinical Trials Start-up and Initiating: The CRA and the site perspective
Responsibilities of the CRA, CTA & CRC during the initial stage of clinical trials: Site Selection, Feasibility Assessment, Pre-study Site Qualification Visit and Site Initiation Visit.
Unfolding all of your opportunities: The Medical Writing Careers in the Clinical Trials Industry: how to get-in
3 KEY ARGUMENTS WHY MEDICAL WRITING CAREERS IN THE CLINICAL TRIALS INDUSTRY ARE OF HIGH INTEREST IF YOU HAVE A PhD OR MD/DVM/BDS/PharmD DEGREE
To enroll with CRA School Montreal, please fill-in the application form by clicking on the ‘Enroll Today’ button.

Workshops
405 Avenue Ogilvy, unit #101
Montreal, QC, Canada H3N 1M3
(legal address, no admission)
Contact Us
info@cra-school.com
Information : +1 (514) 534-0273
Support 7/7 : +1 (514) 257-3003
By Skype: CRA School Montreal
- © 2009 - 2024 CRA-School - International, All Rights Reserved.