
Vishwambari T, MBBS, CCRP. Medical Advisor
Tumorigenesis is fueled by the altered metabolic pathways of necessary nutrients like carbohydrates, lipids and amino acids. These alterations are facilitated not only by carcinogenic proteins, but by non-coding RNAs, among which Circular_RNA (circRNA) has become in the recent years the focus of attention.
CircRNA plays an important role in regulating cellular metabolism through binding with corresponding mRNAs or proteins directly.
We are reviewing the emergent findings and circRNAs contribution to cancer glycolysis and oxidative phosphorylation to understand the cancer metabolic regulatory system. We will also see how circRNA, owing to their unique structure and influence via exosomes and cancer stem cells, may help improving clinical cancer treatment by targeting cancer metabolism.
Background
Deregulated metabolism is a hallmark feature in tumors caused due to genome instability. It is the most important factor in growth and division of cancer cells and hence tumor progression.
The most prominent cancer-associated metabolic changes include the deregulated uptake of glucose and amino acids, use of opportunistic modes of nutrient acquisition, use of glycolysis / tricarboxylic acid cycle (TCA), cycle intermediates for biosynthesis, nicotinamide adenine dinucleotide phosphate (NADPH) production, and increased demand for nitrogen. Research dedicated to studying the underlying abnormal metabolism in cancer is of pivotal importance.
Circular RNA (or circRNA) is a type of single-stranded RNA formed by ‘back-splicing’ of a linear chimeric RNA, it is known to form a covalently closed continuous loop, where the 3′ and 5′ ends, normally present in an RNA, have been joined together. Their circular structure makes them stable in cells and body fluids and resistant to exonuclease digestion, so they have much longer half-life than their parental mRNAs (48 h vs. 10 h). This feature confers numerous properties to circular RNA, many of which have only recently been identified, like its affect on cell growth, cell migration, and its invasion of tumorigenesis. CircRNAs secreted within exosomes are potential cancer biomarkers.
Circular RNAs have a tissue-restricted and cancer-specific expression pattern, and the data collected suggest that these molecules have potential to directly regulate transcription by interacting with mRNAs or long non-coding RNAs (lncRNAs), sponging mRNAs, or RNA binding proteins (RBPs). Some circRNAs can even be translated into proteins.
The aim of this review is to explore the relationship between circRNAs and cancer metabolism to provide a better theoretical basis for clinical diagnosis and treatment of cancers by reviewing information on how circRNAs affect metabolism of carbohydrate, lipid, and amino acids.
CircRNAs in metabolism of Glucose
Cancer cells proliferate by modifying the metabolism of glucose. In norm in aerobic conditions, cells use glucose to generate ATP through oxidative phosphorylation and resort to glycolysis in anaerobic or anoxic conditions. Cancer cells however undergo glycolysis even in normoxic conditions. This is called “the Warburg effect” and it is instrumental in malignancy of cancer cells, as proposed in the 1920s , by Otto Warburg.
This effect alleviates damage due to oxidative stress caused by mitochondria when cancer cells choose glycolysis instead of OXPHOS to generate energy. This is followed by production of excess of lactic acid by the Warburg effect which aids to circumvent the immune surveillance.
The enhanced intracellular transport of glucose in tumor cells, maintains a high-speed glycolysis process and a high level of ATP/ADP ratio. It is hypothesized that the Warburg effect consumes an enormous amount of glucose to either produce excess amount of lactic acid creating a hyperacid microenvironment, or it starves healthy cells in the vicinity of the tumor. The excess amount of lactate produced may also stimulate neo vascularisation via the HIF1-α.. The table below illustrates the effects of circRNAs on aerobic glycolysis of all these levels, circRNAs play a vital role in glycolysis by regulating transporters and enzymes
circRNAs in glycolysis
Table 1:
| circRNA | Mediator miRNA | Target component | Effect on glycolysis | Cancer type |
| circHIPK3 | miR-124 | GLUT2 | Up | – |
| circ-Amotl1 | – | PDK1, AKT1, c-myc, STAT3 | Up | Breast cancer |
| circDENND4C | – | HIF-1α | Up | Breast cancer |
| circ_0010729 | miR-186 | HIF-1α | Up | – |
| circRNA_001569 | miR-145 | C-myc | Up | Colorectal cancer |
| circBIRC6 | miR-145, miR-34a | C-myc | Up | – |
| circ-FBXW7 | – | C-myc | Down | Glioma |
| circNRIP1 | miR149-5p | AKT1 | Up | Gastric cancer |
| circ-ZNF609 | miR-150-5p | AKT3 | Up | – |
| circRNA_103801 | miR-370, miR-877 | PI3K/AKT, HIF | Up | Osteosarcoma |
| circRNA_100290 | miR-29, miR-516b | CDK6, RAS, Wnt/β-catenin | Up | Oral squamous cell carcinoma, Colorectal cancer |
| circRNA-MYLK | miR-29a | RAS | Up | Bladder cancer |
| circ-ITCH | miR-22-3p | CBL | Down | Papillary thyroid cancer |
From: CircRNAs in cancer metabolism: a review
Previous studies have shown that the Warburg effect may be regulated by oncogenes, tumor suppressor genes, and key enzymes in glucose metabolism, but the underlying mechanisms are incomplete. Pieces of evidence show that circRNA is an emerging regulatory factor of the Warburg effect.
CircRNAs in Regulating Transporters of Glycolysis
In the major energy providing pathway, the carbohydrate metabolism pathway involves the participation of a group of metabolic enzymes. The process of glycolysis starts when glucose transporter (GLUT) transports extracellular glucose into the cell. To meet the excessive requirements of glucose, GLUTs ranging from GLUT1 to GLUT4 are all over-expressed in tumor cells.
Studies have shown that certain circRNAs are significantly up-regulated in the tumor tissues, which in turn promotes tumor glycolysis, growth, and metastasis by affecting the cell metabolism. The suppression of circHIPK3 in pancreatic islets can reduce the expression of Slc2a2, which encodes GLUT2. CircHIPK3 reduces the expression levels of transporters as well as enzymes, and inhibits glycolysis by targeting miR-124. Circ-Amotl1 can target AKT1 and PDK1.
The down-regulated circARHGAP10 in non-small cell lung cancer(NSCLC) can decrease the expression of GLUT1, which inhibits intracellular transport of glucose, glucose consumption as well as lactate formation this consecutively inhibits glycolysis by sponging miR-150-5p. In NSCLC, hsa_circ_0002130 targets miR-498 to up-regulate GLUT1, hexokinase 2 (HK2), and lactate dehydrogenase A (LDHA), regulating the glycolysis and eventually progresses NSCLC by increasing the resistance towards Osimertinib.
In colorectal cancer (CRC), the absorption of miR760, regulation of GLUT1, and stimulation of glycolysis, growth, as well as metastasis of cells. is directly influenced by the up-regulated circDENND4C. On the other hand, down-regulation of circDENND4C can inhibit glycolysis in CRC cells, and overexpression of GLUT1 can remedy this inhibition.
In oral squamous cell carcinoma (OSCC), the expression of circRNA_100290 cells was significantly up-regulated. GLUT1 was up-regulated through miR-378a to promote glycolysis, growth, and metastasis of cells.
CircRNAs in Regulating Enzymes or Kinases of Glycolysis
CircRNAs also effect the enzymes of glucose metabolism. Another circRNA called circ-Amotl1 was reported to be able to physically bind to PDK1 and AKT1 and translocate them into the nucleus, which antagonizes apoptosis. Three speed-limiting enzymes including hexokinase (HK), 6-phosphfructa-1-kinase (PFK), and pyruvate kinase (PK) work together to transform glucose into pyruvate. Lactate dehydrogenase A (LDHA), which is the final enzyme in aerobic glycolysis, catalyses pyruvate to lactate.
Recent studies report that in the production of lactic acid from pyruvate, some circRNAs are able to influence the glycolysis by targeting ENO1, PKM2, and LDHA. Eventually, the monocarboxylate transporter (MCT) releases this lactate into the extracellular matrix. In addition to this, pyruvate dehydrogenase kinase (PDK) blocks the traditional OXPHOS by neutralising pyruvate dehydrogenase (PDH) which catalyses pyruvate into acetyl-COA.
PDK1 is a component of the PI3K/AKT pathway to affect cell growth, differentiation, and survival. AKT1, as a protein kinase signaling pathway known to trigger cancer, regulates the expression of transcription factors (TFs) and affects cellular glycolysis by activating downstream cell growth regulators.
Some experimental studies have shown that suppressing circHIPK3, which is abundant in pancreatic islets, decreased Slc2a2 expression that encodes GLUT2. CircHIPK3 could sponge miR-124, which represses the expression of several enzymes and transporters of glycolysis. CircRNA circ-Amotl1 is capable of physically binding to PDK1 and AKT1 and translocate them into the nucleus, which antagonises apoptosis. These circRNAs are likely to induce the Warburg effect in tumor tissues.
ENO1
Enolase 1 (ENO1), plays an important regulatory role in the Warburg effect of tumor cells.
Circ-ENO1 can increase the expression of ENO1 in Lung Adenocarcinoma (LUAD) by targeting miR-22-3p and the suppression of circ-ENO1 reduces the activity of ENO1, delay the uptake of glucose and formation of lactic acid, and eventually inhibit cell glycolysis. Reduced ATP levels, EMT, inhibition of cell growth, cell migration, and apoptosis are induced by down-regulated circ-ENO1.
PKM2
Pyruvate kinase (PK) is a crucial enzyme in both normal glycolysis as well as in abnormal glycolysis during tumorigenesis. PK plays a crucial role in the final step of glycolysis where it catalyses the conversion of phosphoric acid by phosphoenolpyruvate and ADP to ATP.
There are 4 isozymes of pyruvate kinase namely-PKXL, PKR, PKM1 AND PKM2, each of these are expressed during gluconeogenesis in different organs. PKM2 which is expressed in the embryonic cells, stem cells, and tumor cells with active nucleic acid syntheses.
PKM2 also has a vital role in cancer growth and carbohydrate metabolism in tumor cells. PKM2 exists in two isomeric forms: a highly active tetramer and a low-activity or restrictive dimer. The dimeric form promotes the anabolism of carbohydrates, and the tetrameric form aids in OXPHOS of glucose and provides energy for cells. In tumor cells the overexpression of PKM2 and the transformation between the two forms aids the tumor cells survival in various environments.
In Colorectal Carcinoma (CRC), hsa_circ_0005963 (ciRS-122) exosomes are transported from oxaliplatin-resistant cells to sensitive cells. the reduced ciRS-122, targets miR-122 and up-regulates the expression of PKM2 in oxaliplatin-resistant cells, which boosts aerobic glycolysis, ATP production, and the development of CRC.
LDHA
Lactate dehydrogenase A (LDH-A) executes the last step of aerobic glycolysis and catalyses the inter-conversion of pyruvate and L-lactate. In bladder cancer (BC) the prognosis is poor when, in cells experiencing hypoxic environment, aerobic glycolysis is promoted by the up-regulated has_circRNA_403658, which primarily regulates the expression of LDHA. The excess production of has_circRNA_403658 in triggered by excess of HIF1 and is encoded by its host gene ZNF292. Similarly when the production of has_circRNA_403658 was stifled there was a significant down regulation of LDHA leading to reduced LDH activity, lactate production, glucose absorption, along with ATP production.
Transcription factors, Genes, and Signaling Pathways of Glycolysis
CircRNA is a member of the ncRNAs and has an important role in monitoring the expression of mutation of Transcription factors (TF) and their regulatory function. The circRNA achieves this by targeting the HIF-1, C-myc, CUX1, p53, FLOT2, RAS, and STAT3, which are the TF of miRNAs, there by eventually modulating the miRNAs. These TFs also have impact on the Warburg effect.
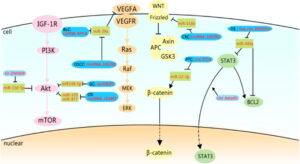
Signaling Pathways of Glycolysis
Fig 1: Relationships between circRNAs and TFs
HIF-1
The Warburg effect is directly proportional to the hypoxia in the cell. Activation of oncogenes in hypoxic solid tumor cells result in transcription of genes and enzymes like HK, PKM, MCT4 abd Hypoxia-inducible_factor HIF-1, which encourages glycolysis instead of OXPHOS. This increases the production of lactate proportional to the level of HIF-1 by up-regulating PDK1
In breast cancer cells, HIF-1α can up-regulate circDENND4C. This can target the miR-200b and miR-200c, which eventually down-regulates LDHA and Sirtiuin 2. This inhibits tumor glycolysis, and insistently promotes cell proliferation in a hypoxic environment. In human umbilical cord vascular endothelial cells (HUVEC), circ_0010729 is over expressed along with HIF-1, especially in hypoxia-induced HUVEC.
By sponging miR-186, this over-expressed circ_0010729 promotes glycolysis and cell growth, and up-regulating HIF-1α. This causes increased glucose supply to the tumor cells with the support of new vessels. Hence, it can be said that circ_0010729 facilitates glycolysis by accelerating the development of vascular endothelial cells
C-Myc
C-Myc is one of the most crucial oncogenic TFs. It regulates cell metabolism and can also activate the Warburg effect. The collaboration between C-Myc and over expressed HIF-1 is known to raise the levels of glycolytic components GLUT1, HK2, and PDK1. C-myc is an oncogene, which can be regulated by several circRNAs.
In CRC, miR-145 is targeted by both circRNA_001569 and circBIRC6. Certain circRNA have the ability to target more than one miRNAs like circBIRC6 also targets miR-34a along with miR-145. miR-34a has the ability to inhibit c-myc oncogene. Circ-Amotl1 can also supress the nuclear translocation mutation of c-myc, and also influence the STAT3 and AKT which in turn promotes tumor development through enhanced aerobic glycolysis and not by using endogenous RNA (ceRNA).
Interestingly, a protein FBXW7-185aa which is capable of interacting with de-ubiquitinating enzyme USP28, could be encoded by circ-FBXW7. This circ-FBXW7-185aa, could possibly indirectly degrade c-myc thereby ameliorating the Warburg Effect.
P53
The tumor suppressor gene P53 is an important TF which can regulate tumor metabolism along with HIF-1 and C-Myc oncogene, as a tumor suppressor gene. P53 helps circRNA in regulating metabolism. In triple negative breast cancer (TNBC), research has shown that circSEPT9 has the ability to regulate the expression of Leukemia Inhibitory Factor (LIF) via sponging miR-637 and activate LIF/Stat3 signaling pathway involved in progression of TNBC. The elevated levels of circSEPT9 which was found in TNBC tissues, is and indicator of poor prognosis due to advanced clinical stage.
On the other hand, suppression of circSEPT9 in TNBC cells reduced proliferation, migration and invasion of cells, stimulated apoptosis and autophagocytosis in TNBC cells, as well as inhibited tumor growth and metastasis in vivo. It can be concluded that, the potential of circSEPT9 as a prognostic marker and therapeutical target for TNBC is worthy of further research.
Regulation of signaling pathways
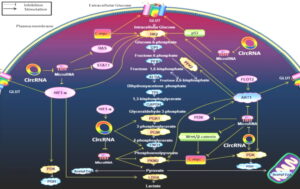
The effects of circRNAs
Fig 2: The effects of circRNAs on the Warburg effect-associated signaling pathways. This figure indicates the effects of circRNAs on the Warburg effect-associated signaling pathways. It shows how circRNAs interact with these metabolic pathways by enzymes and transporters associated with the Warburg effect.
The majority of circRNAs can antagonize microRNAs to indirectly produce effects. HIF1-α regulates GLUT, HK2, PDK, LDHA. The PI3K/Akt pathway promotes glucose GLUT, HK2, PFK2, pyruvate kinase M2 (PKM2), and PDK. The change of RAS pathway up-regulated HK2. P53 up-regulated GLUT, HK2. The STAT3 pathway promotes HK2. Wnt/β-catenin pathway up-regulates PDK transcription while promoting c-myc. The positive relationships are shown by arrows, and the negative relationships are shown by short dashes.
The collective work of all TFs forms a network, which consists of various signalling pathways involving diverse circRNAs. This regulatory pathway is known to affect the Warburg effect.
PI3K/Akt
PI3K/Akt pathway is vital in glycogenesis and promoting glucose uptake. Glycogenesis is stimulated by HK2 and PFK2 under the influence of Akt (Protein_kinase_B). PI3K/Akt signaling pathway, affects the absorption of glucose and glycogenesis by increasing the antioxidant activity of HIF-1α along with the elevated levels of HK2 and PFK2. Down streaming of Akt1 is brought about by MTOR complex1, which can phosphorylate elf4E-binding proteins and support HIF-1α to promote GLUT1, HK2, and PFK2.
In Hirschsprung disease, he levels of Akt# and circ-ZNF609 are elevated, while the level of miR-150-5p expression is reduced. It can be said circ-ZNF609 might support AKT3 by targeting miR-150-5p which improves the Warburg effect. More research is required to confirm its role in cancer metabolism.
RAS
The KRAS gene provides instructions for making a protein called K-Ras (Kirsten rat sarcoma virus) that is part of a signaling pathway known as the RAS/MAPK pathway. The KRAS belongs to a class of genes known as oncogenes. These proteins play important roles in cell division, cell differentiation, and apoptosis. When mutated, they become cancerous, especially in colorectal cancer, non-small cell lung cancer, and prostrate cancer. Mutated KRAS upregulates the GLUT1 , and glycolytic enzymes thereby contributing to the Warburg effect in cancer cells.
STAT3
The Signal Transducer and Activator of Transcription 3 (STAT3) is a transcription factor which in humans is encoded by the STAT3 gene. STAT3 is studied to be very important in the development, progression, and maintenance of many human tumors, validating STAT3 as an anticancer target. The STAT3 pathway promotes inflammatory environment, cell proliferation which favors cancer development under the stimulation by circ-Amotl1.
In osteosarcoma, miR-449a mediates the STAT3 pathway indirectly with the aid of hsa_circ_0009910. This hsa_circ_0009910 can have a sponging effect on miR-449a which antagonises IL6R.
Wnt/β-catenin
Wnts are glycoproteins which act as ligands in stimulating receptor-mediated signal transduction pathways, which modulate cell proliferation, survival, and cell behavior. Upregulating the transcription of MCT1 and PKD1 is how the Wnt/β-catenin pathway regulates the Warburg effect.
In NSCLC cells, the circCDR1as/miR-219a-5p/SOX5 axis may target the Wnt/β-catenin pathway and regulate the Warburg effect. In nasopharyngeal carcinoma (NPC), circCDR1 targets the miR-7-5p, upregulates E2F3, which increases the glucose consumption and production of lactic acid.
In contrast, suppression of circCDR1as, triggers the Wnt/β-catenin pathway to significantly inhibit the metabolism of HK1 and HONE1 cells (NPC line).105
All in all, circRNAs that regulate tumor cell metabolism of lung adenocarcinoma and nasopharyngeal carcinoma are closely related to the Wnt/β-catenin pathway.
Conclusion
In depth research in the field of circRNA has brought forward a lot of new understandings about cancer glucose metabolism. Evidence that Warburg effect may be triggered by the rogue circRNAs, which may contribute to development of various malignancies by impacting various metabolic pathways in cancer.
There are studies showing evidence of close association between circRNA and carbohydrate, lipid, and amino acid metabolisms. Glucose metabolism is a topic of review herein, where the molecular mechanism involves circRNA in tumor glycolysis by regulating transporters, enzymes, kinases, TFs, genes, and glycolysis signaling pathways (Figure 2) was reviewed.
It is worth investing further how the abnormally expressed circulation circRNAs participate in cancer glucose metabolism, as they are substantially related to various cancer types in humans
Vishwambari Tamary, MBBS, CCRP,
Medical Advisor and Consultant at the
International Clinical Research Academy
The “CRA-School” of Montreal
References
- CircRNAs in cancer metabolism: a review: Tao Yu, Yanfen Wang, Yu Fan, Na Fang, Tongshan Wang, Tongpeng Xu& Yongqian Shu Journal of Hematology & Oncology ( https://link.springer.com/article/10.1186/s13045-019-0776-8#Fig2 )
- Tip of the Iceberg: Roles of CircRNAs in Cancer Glycolysis Tan Li,1Hong-chun Xian,2 Li Dai,1 Ya-lingTang,2 and Xin-huaLiang1 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8039208/)


