Welcome to Our Blog
The Principal Investigator role in Women Health Clinical Trials, free webinar with Dr Janet Gerstnen
The Principal Investigator role - responsibilities and challenges in Women Health Clinical Trials...
The LMC Manna Research Covid-19 vaccine clinical trial, free webinar with Jacqueline Nguyen, CRC
Covid-19 vaccine clinical trial of LMC Manna Research - Study organization from a site...
The MSL role – 10 myths and exciting opportunities, free webinar with Dr Plamen Stoychev, MD, MSL
The MSL (Medical Science Liaison) role - an excellent opportunity for foreign-trained IMGs,...
Understand 8 rewarding Medical Affairs Jobs, free webinar with Naila Inam, Sr MSL manager at Amgen
How to become an MSL, Drug Safety or Pharmacovigilance specialist, Medical monitor, Medical...
One rewarding day in the life of a CRA – tasks and challenges, free conference of Ken Boudrault, SrCRA
Join the Conference of Ken Boudrault, SrCRA & mentor See 1 exciting day in the life of...
Covid-19 hastens the need for new clinical trials, staff & sites, both in the industry and in academic settings
Interested to work in Clinical Trials as a Clinical Research Assistant or Clinical Research...
Clinical Trial Workshop with Ken: Clinical Trials Start-up and Initiating: The CRA and the site perspective
Clinical Trial Start-up and Site Initiation process from the CRA perspective - The number 1...
Unfolding all of your opportunities: The Medical Writing Careers in the Clinical Trials Industry: how to get-in
Are you MSc/PhD or MD/PharmD ? Uncover your full potentialby getting a rewarding career in...
Pharma Regulatory Affairs Jobs – How to break in – Global networking event. Talk to an expert
Pharma Regulatory Affairs Jobs - How to break in - Global networking event series. Talk to an...
Réglementation Algérienne sur la recherche clinique, avantages et défis
Les essais cliniques en Algérie Le pays compte plus de 200 établissements de santé,...
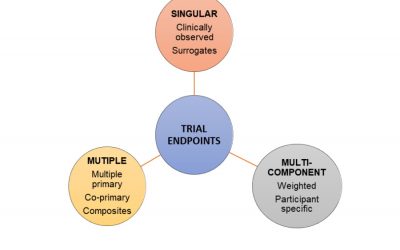
The Endpoint Selection: a Complex Process in the Clinical Trials Design
Endpoint selection: a complex process in Clinical Trials End-users of clinical trials such as...
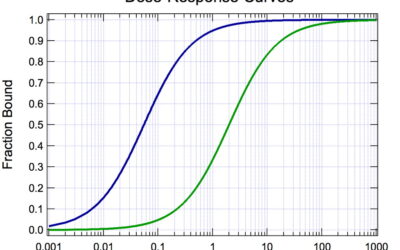
Pharmacometric modelling and simulation: an important tool in therapeutic decision-making
Pharmacometric modelling and simulation: a tool in therapeutic decision-making When a medicine...
To enroll with the CRA School Montreal, please fill in the application form by clicking on the ‘Enroll Today’ button.
Clinical Research Advisor
Ken Boudreault,
Senior CRA II
Synteract, Montreal
Consultant & Mentor
20+ years experience
Data Management Advisor
Nassima Benmouffok
Ass. Director of Data Management,
Ethica CRO, Montreal
Senior Data Manager
Medical Science Liaison Advisor
Thomas Rolain,
PhD, CCRP/CCRA
Syneos Health
SrCRA and MSL
Pharmacovigilance and Drug Safety Advisor
Medical Writing Advisor
Sefika Ozturk, PhD
Medical advisor
MSL at Merck
Clinical Trials Management Advisor
Radia Ouelaa, PhD
Clinical Team Manager, PPD
Onco/Hematology
Clinical Research Management
Janet Vergara, SrCRA
Ass.Director at Merck
Clinical Research Manager
Clinical Trials Advisor, Trainer and dedicated Mentor
Bahareh Najjar SrCRA
MedPace, Montreal
CRA Mentor and Trainer
Clinical Operations Advisor
Anna Leonov, PhD
Clinical Operations
Syneos Health
Regulatory Affairs Advisor and Mentor
Mona Ramezani
IH SrCRA at Merck
Regulatory Affairs
Medical Devices Advisor
Monir El Azzouzi,
CEO and Consultant
Easy Medical Device
Medical Writing Advisor and dedicated Mentor
Dr Anand Devasthanam, PhD
Ass. Medical Director
Syneos Health, Boston
France
Karima Kezadri, MBA, MSc, CCRP
Regional Rep. for
Maghreb & MENA
Tel: +213559918058
hopekarima@yahoo.fr
Nigeria
David Dele-Davids
Lagos, Nigeria.
Tel:+2349093309249
WhatsAp: +19495202173
deledavidsconsulting@gmail.com
India
Monisha Sundar
Ontario; Canada
New Delhi; India
Tel: +16472387293
+918427688701
mishu_shiv@hotmail.com
Bulgaria
Dr. Jamil Rashed
Sofia, Bulgaria.
Tel: +359879450847
Djamilrashed@gmail.com
AUSTRALIA
Jyothi Surada,
BPharm, CCRP
CRC,
BC
Benard Kimwei
Burnaby, BC
Tel: +1 403 465 6900
benard.kimwei@gmail.com
AB
Olaniyi Taiwo
Edmonton, AB
Tel: +1 306 450 2974
sotaiwo06@gmail.com
MB
Dina Tabatabaei, MA,
CCRP, CRA School Rep
Brandon, MB
(416)879-7575
dtabataba88@gmail.com
USA
David Dele-Davids
Baltimore, MD, USA.
Tel: +1(949)520-2173
deledavidsconsulting@gmail.com
UGANDA
Daniel Okodan, MSc,
CCRA, CRA at PPD
Entebbe, Uganda
okodandaniel@gmail.com
DUBAI
Taline Sagherian, RD
CCRP, Dubai, UAE
+(971) 50 935 4208
sagheriantaline@gmail.com
FIJI
Oluwafemi Ojo, MD, CCRA
Physician, Tawua, Fiji
drfemiojo@gmail.com
GERMANY
UGANDA
Sylvia Namanda,
BSN, CCRP,
Makarere University
(+256) 756 762 005
sylvian531@gmail.com
IVORY COAST
ISSATOU DIALLO, CRA
Regional Manager
Afrika at MCT-CRO
issatoubella@gmail.com
Medical Writer
Christina Sanguinetti,
Medical Writer, CEO
LaconicMed, ON
christina@laconicmed.ca

Workshops
405 Avenue Ogilvy, unit #101
Montreal, QC, Canada H3N 1M3
(legal address, no admission)
Contact Us
info@cra-school.com
Information : +1 (514) 534-0273
Support 7/7 : +1 (514) 257-3003
By Skype: CRA School Montreal
- © 2009 - 2024 CRA-School - International, All Rights Reserved.